When you pick up a prescription at the pharmacy, you might see two options: the familiar brand-name pill, or a cheaper generic version with a different color and shape. You’ve probably wondered - is the generic just as good? The answer lies in something called bioequivalence testing. It’s not marketing. It’s science. And it’s the reason millions of people safely use generic drugs every day.
What Bioequivalence Testing Actually Measures
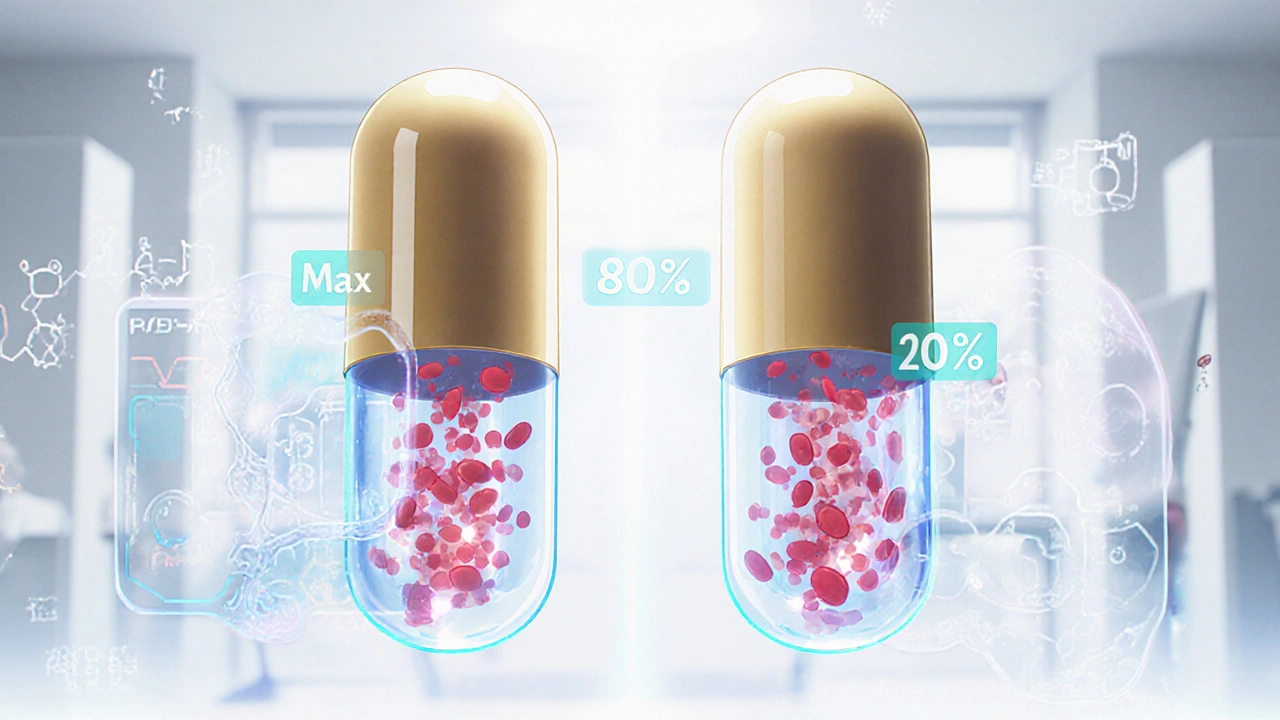
Bioequivalence testing doesn’t check if a generic drug works the same in a test tube. It checks how your body handles it. Specifically, it measures two things: how fast the drug gets into your bloodstream (rate), and how much of it gets in overall (extent). These are called Cmax and AUC.For a generic drug to be approved, its Cmax and AUC must fall within 80% to 125% of the brand-name drug’s values. That’s not a wide range - it’s tight. It means if the brand drug hits 100 units of concentration in your blood, the generic can’t be below 80 or above 125. This isn’t guesswork. It’s based on decades of data and statistical analysis.
The test is done in healthy volunteers - usually 24 to 36 people - who take both the brand and generic versions in a crossover design. One week, they take the brand. The next week, they take the generic. Blood samples are taken at regular intervals to map out how the drug moves through the body. This design eliminates individual differences because each person is their own control.
This is how the FDA and EMA ensure that a generic drug isn’t just chemically identical - it behaves the same inside your body.
Why This Matters More Than You Think
Most people think a generic drug is just a copy of the brand. That’s not quite right. The active ingredient? Same. The dose? Same. But the fillers, dyes, coatings? Different. Those inactive ingredients can affect how quickly the pill breaks down in your stomach. That’s why bioequivalence testing is non-negotiable.Take a blood pressure pill. If the generic releases the drug too slowly, your pressure might spike. Too fast? You could get dizzy. Bioequivalence testing catches those differences before the drug ever hits the shelf.
And it works. In 2020, generic drugs saved the U.S. healthcare system $313 billion. They make up 90% of prescriptions but only 23% of drug spending. That’s not luck. That’s science working as intended.
How It’s Different From Brand Drug Approval
Brand-name drugs go through years of clinical trials - hundreds, sometimes thousands of patients - to prove they’re safe and effective. Generic manufacturers don’t repeat those trials. Why? Because they don’t need to.The FDA already knows the brand drug works. The generic just has to prove it gets into your body the same way. That’s the whole point of the Abbreviated New Drug Application (ANDA) pathway. It cuts red tape without cutting corners.
The process still takes 10 to 12 months. About 6 to 8 of those months are spent on bioequivalence studies. The FDA inspects the manufacturing plant. They check the dissolution profile - how the pill breaks down in simulated stomach fluid. They verify purity and stability. And they don’t just approve one batch. They approve the whole production line.
That’s why a generic drug made in India or China can be just as reliable as one made in the U.S. - if it passes the same tests.
Where Bioequivalence Testing Falls Short
Not all drugs are created equal. Some are complex. For example, inhalers, topical creams, and eye drops don’t always get absorbed into the bloodstream the same way pills do. You can’t just measure blood levels and call it done.For inhalers, regulators look at how well the drug reaches the lungs - not how much shows up in your blood. For topical steroids, they check if the cream penetrates the skin enough to work without causing side effects. These require different kinds of testing: clinical endpoint studies, pharmacodynamic measurements, or even patient-reported outcomes.
Drugs with a narrow therapeutic index - like warfarin, lithium, or levothyroxine - are especially tricky. A tiny difference in absorption can mean the difference between no effect and a dangerous overdose. For these, the FDA sometimes tightens the bioequivalence range to 90-111% instead of 80-125%. And they may require additional studies.
That’s why some doctors still hesitate to switch patients on these drugs. Not because the science is flawed - but because the margin for error is razor-thin.
What Patients Really Experience
A 2022 Consumer Reports survey of 1,200 people found that 87% saw no difference between their generic and brand-name drugs. Nine percent said the generic worked better. Only 4% said it worked less effectively.On Reddit’s pharmacy forums, 78% of users who shared experiences reported no change in how their medication worked after switching to generic. The complaints? Mostly about side effects from new fillers - like stomach upset from a different dye or coating. Not lack of effectiveness.
Still, myths persist. A 2021 study found 32% of patients believe generics are less potent. They think generics “take longer to work” or “aren’t as strong.” But bioequivalence testing proves otherwise. If the drug gets into your blood at the same rate and amount, it will work the same way.
One common confusion: if you feel worse after switching, it’s not because the drug is weaker. It’s because your body is adjusting to a different filler. That’s temporary. Talk to your pharmacist. Don’t assume the generic failed.
The Bigger Picture: Global Standards and Future Trends
Bioequivalence isn’t just an American thing. The European Medicines Agency, Health Canada, Japan’s PMDA, and others follow nearly identical standards thanks to the International Council for Harmonisation (ICH). That’s why a generic made in Germany works the same in the UK as one made in the U.S.The global generic drug market is growing fast - projected to hit $781 billion by 2030. Why? Because patents are expiring. Drugs like Humira, Enbrel, and others are opening up to competition. More generics mean lower prices. More access.
And the science is evolving. The FDA is now exploring computer modeling to predict how a drug behaves in the body - called PBPK modeling. Instead of testing 30 people, maybe you test 5 and simulate the rest. It’s faster, cheaper, and still accurate.
For complex drugs like inhalers, regulators are pushing for real-world evidence - tracking how patients do over time after switching. This isn’t replacing bioequivalence. It’s adding to it.
Bottom Line: Trust the Science
Bioequivalence testing isn’t perfect. But it’s the best tool we have. It’s rigorous. It’s transparent. It’s backed by decades of data and global regulatory consensus.Generic drugs are not second-rate. They’re science-backed alternatives that save billions and make healthcare accessible. If your doctor prescribes a generic, or your pharmacy fills it automatically - you’re not taking a risk. You’re benefiting from one of the most successful public health policies in modern medicine.
Don’t let myths scare you. If the FDA says it’s bioequivalent, it is. The numbers don’t lie.
Are generic drugs really as effective as brand-name drugs?
Yes. Generic drugs must prove bioequivalence to their brand-name counterparts by showing identical absorption rates and total exposure in the body. The FDA requires that the 90% confidence interval for key measurements (AUC and Cmax) fall between 80% and 125% of the brand drug’s values. This ensures they work the same way in your body.
Why do generic pills look different from brand-name pills?
By law, generic drugs can’t look identical to brand-name drugs - that would violate trademark rules. So manufacturers change the color, shape, or coating. But the active ingredient, strength, and dosage form must be the same. These cosmetic differences don’t affect how the drug works.
Can I trust generics made outside the U.S.?
Yes. The FDA inspects over 1,200 manufacturing facilities worldwide each year - including in India, China, and Europe. A generic drug must meet the same quality, purity, and stability standards regardless of where it’s made. If it’s approved by the FDA, it’s held to the same standard as U.S.-made drugs.
Why do some people say generics don’t work as well?
Most reports of reduced effectiveness are due to side effects from inactive ingredients - like dyes or fillers - not the active drug. Some people are sensitive to these, leading to stomach upset or minor reactions. This isn’t a failure of bioequivalence. It’s a personal tolerance issue. Switching to a different generic or the brand may help, but the drug’s potency remains unchanged.
Are there drugs where generics shouldn’t be used?
For most drugs, generics are safe and effective. But for narrow therapeutic index drugs - like warfarin, levothyroxine, or lithium - doctors may be cautious. Even small changes in absorption can cause problems. In these cases, the FDA may require tighter bioequivalence limits or additional testing. Always consult your doctor before switching.
How long does it take to get a generic drug approved?
The FDA’s average review time for a generic drug application (ANDA) is 10 to 12 months. About 6 to 8 months of that time is spent reviewing the bioequivalence study data. Under the GDUFA program, 95% of applications are reviewed on time, with no compromise in safety or quality standards.
Do bioequivalence tests guarantee the same side effects?
Bioequivalence testing only confirms that the active ingredient behaves the same in the body. It doesn’t predict side effects from inactive ingredients. That’s why some people report new reactions after switching - like nausea or rash - due to different dyes or binders. These are rare and usually mild. If they occur, talk to your pharmacist about trying a different generic version.
What’s the difference between bioequivalence and therapeutic equivalence?
Bioequivalence means the drug is absorbed the same way. Therapeutic equivalence means it’s safe and effective for the same use. The FDA assigns therapeutic equivalence codes in the Orange Book. Drugs rated AB1 or AB2 are considered interchangeable. Not all bioequivalent drugs are rated therapeutically equivalent - especially if they have different delivery systems or are not approved for all the same uses.


Comments (12)