When a doctor writes a prescription, they’re not just choosing a drug-they’re choosing a treatment path. And for most patients, that path leads to a generic medication. Over 90% of prescriptions in the U.S. are filled with generics. But do they work the same? That’s the question providers keep asking, especially when a patient comes back saying, "This one doesn’t feel right." The answer isn’t opinion. It’s in the data.
How Generics Are Approved: It’s Not What You Think
Many assume generics are "cheap copies." That’s wrong. The FDA doesn’t let a generic drug onto the market unless it matches the brand-name drug in every meaningful way. The active ingredient? Identical. The dose? Exact. The way it’s taken? Same pill, same injection, same patch.
The real test is bioequivalence. That means the body absorbs the drug at the same rate and to the same extent. For most drugs, the FDA requires that the generic delivers between 80% and 125% of the brand’s concentration in the bloodstream. That’s not a wide margin-it’s a tight one. And it’s not based on theory. It’s proven in clinical studies with 24 to 36 healthy volunteers. The area under the curve (AUC) and peak concentration (Cmax) are measured. If the numbers land in that range, the drug is approved.
For drugs with a narrow therapeutic index-like warfarin, levothyroxine, or tacrolimus-the rules are even stricter. The FDA uses Scaled Average Bioequivalence (SCABE), which accounts for how much the drug varies within a single person. A 2020 study in Nature Scientific Reports tracked transplant patients switching between brand and generic tacrolimus over 42 days. No clinically meaningful differences in blood levels or rejection rates were found.
What the Real-World Studies Say
Thousands of studies have compared generics and brand-name drugs in actual patients-not lab settings. The most comprehensive came from PLOS Medicine in 2019. Researchers looked at 14 different clinical outcomes across seven drug classes. They matched over a million patients, adjusting for age, income, comorbidities, and more. The results? For 12 out of 16 comparisons, there was no statistically significant difference between generic and brand-name drugs.
- Alendronate (osteoporosis): Fracture rates were identical (HR 1.00).
- Glipizide (diabetes): Rates of insulin initiation due to poor control were the same (HR 1.01).
- Quinapril (blood pressure): Hospitalizations for heart attack or stroke were nearly identical (HR 0.99).
- Amlodipine (blood pressure): Generics actually had better outcomes (HR 0.91).
Cardiovascular drugs showed the strongest evidence of equivalence. A 2008 meta-analysis of 47 studies found 89% showed no difference in effectiveness or safety. Even more telling: patients who switched from brand to generic were no more likely to go back to the brand. The FDA tracked this across 12 drugs. The switch-back rate? Just 2.7% for generics. For authorized generics (made by the brand company), it was even lower-1.8%.
The Exceptions: Where Providers Should Pause
There are cases where patients report differences. And while population-level data says they’re equivalent, individual experiences matter.
Psychiatric drugs are the most commonly cited. The PLOS Medicine study found slightly higher psychiatric hospitalization rates with generic escitalopram (HR 1.05) and sertraline (HR 1.07). But here’s the catch: the same pattern appeared when comparing authorized generics to brand-name drugs. That means the difference isn’t about the generic being inferior-it’s about perception. Patients expect a different pill to work differently. That expectation can trigger nocebo effects-where belief in worse outcomes actually causes them.
Also, 3% of generics are rated "B" by the FDA’s Orange Book. These are drugs where bioequivalence is harder to prove: inhalers, topical creams, or complex formulations. For these, providers should monitor more closely. A patient switching from a brand inhaler to a generic might need extra coaching on technique, since the device mechanics can differ-even if the drug inside is identical.
Cost Isn’t Just a Number-It’s a Clinical Factor
Generics cost 80-85% less than brand-name drugs. That’s not just a savings for insurers. It’s a lifeline for patients.
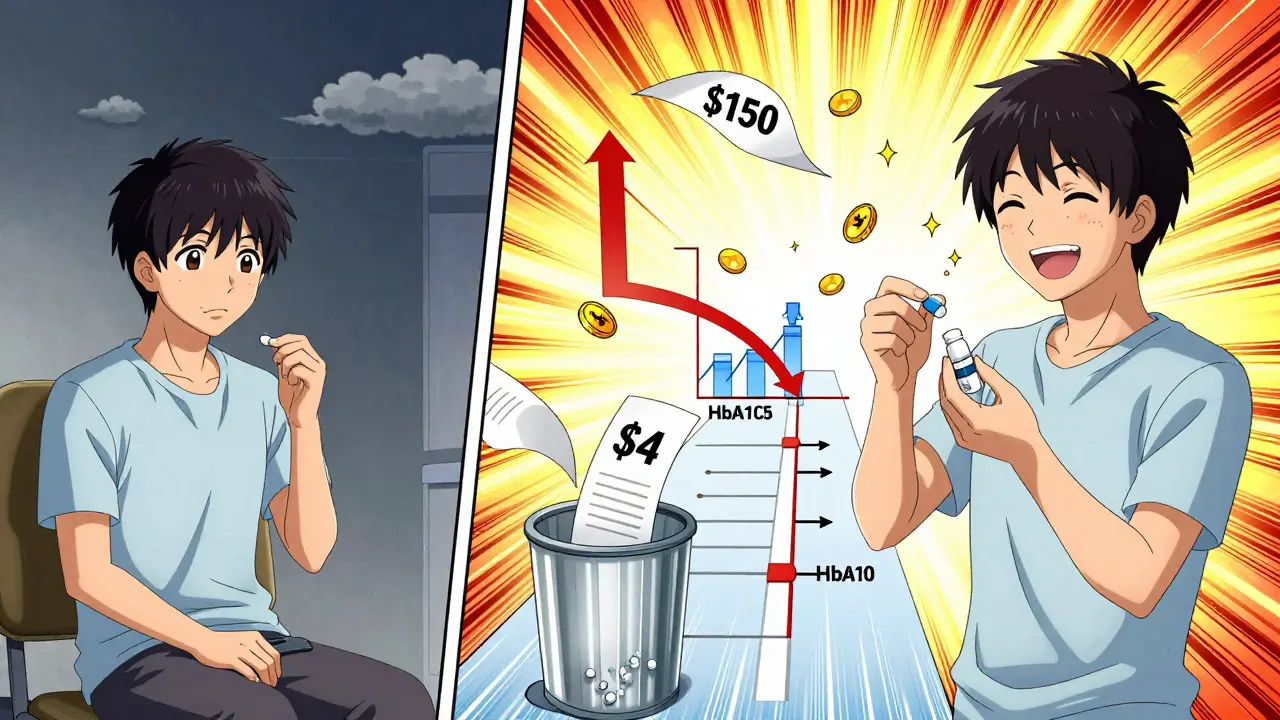
Consider this: a patient on brand-name metformin might skip doses because of the $150 monthly cost. Switch to the generic-$4-and they take it consistently. A 2023 study of 2.1 million diabetics found identical HbA1c control between generic and brand metformin. But the real win? The generic group had fewer emergency visits and hospitalizations because they stayed on therapy.
The Congressional Budget Office estimates generics saved $1.68 trillion from 2008 to 2017. In 2021 alone, they saved the system $377 billion. That’s not accounting for the ripple effect: fewer hospitalizations, fewer complications, fewer missed workdays. When a patient can afford their meds, outcomes improve-even if the pill looks different.
Why Patients Doubt Generics (And How to Address It)
Patients notice the difference. The shape. The color. The imprint. They’ve been conditioned to believe that if it doesn’t look like the brand, it’s not the same.
One study found that 30% of patients believed generics were less effective, even after being told they were identical. The FDA’s own 2019 review confirmed that appearance changes don’t affect clinical outcomes-but they do affect trust.
Here’s what works: Don’t assume they know. Say it plainly: "This generic has the same active ingredient as the brand. It’s been tested in thousands of people and approved by the FDA. The only difference is the price." Show them the FDA’s website. Point out the same active ingredient listed on the label. If they’re still uneasy, offer the authorized generic-same manufacturer, different label.
Also, document the conversation. If a patient insists on the brand, note it. But don’t let cost barriers go unchallenged. A $200 copay isn’t just a financial burden-it’s a treatment failure waiting to happen.
The Bottom Line for Providers
The data is clear: for the vast majority of patients, generics are just as safe and effective as brand-name drugs. The FDA, Harvard, Stanford, and the American College of Physicians all agree. The evidence is overwhelming.
That doesn’t mean you ignore patient concerns. It means you meet them with facts-not assumptions. You don’t have to convince everyone. But you do have to make sure cost doesn’t become the reason someone doesn’t get better.
Prescribe the generic. Talk about it. Track outcomes. And remember: the best drug is the one the patient takes.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generics to meet the same strict standards for quality, purity, and strength as brand-name drugs. They use the same active ingredients and are manufactured in the same types of facilities. Adverse event reports show only 0.02% of all drug-related reports involve generics-far lower than the 3.2% for brand-name drugs. The difference isn’t safety-it’s perception.
Why do some patients say generics don’t work for them?
In most cases, it’s not the drug-it’s the expectation. Patients often associate the brand’s appearance with effectiveness. Switching to a different-colored pill can trigger anxiety, which may lead to perceived side effects or reduced effectiveness. Studies show that even authorized generics (made by the same company as the brand) show similar patterns, proving it’s about perception, not performance. Open conversations and clear explanations help.
Do generics take longer to work?
No. Bioequivalence testing ensures generics reach the bloodstream at the same rate and in the same amount as the brand. If a brand drug peaks in 2 hours, the generic must too. For drugs with extended-release formulas, the release profile is tested to ensure consistency. Any delay in effect is usually due to individual metabolism or non-adherence-not the drug itself.
Are there any drugs where generics aren’t recommended?
The FDA rates 97% of generics as "A"-therapeutically equivalent. The remaining 3% are "B-rated," meaning bioequivalence couldn’t be fully confirmed. These are usually complex products like inhalers, topical ointments, or certain injectables. For these, providers should monitor patients closely. But even here, most "B-rated" generics still work well. The key is to use clinical judgment and patient feedback-not blanket rules.
Can I switch a patient from brand to generic safely?
Yes, for the vast majority of medications. The FDA and multiple large studies confirm that switching between brand and generic drugs does not increase risk of adverse events. In fact, switching to generics often improves adherence and long-term outcomes because of lower cost. For narrow therapeutic index drugs, monitor levels briefly after the switch-but don’t assume a problem exists just because the pill looks different.




Comments (14)