Pharmacists don’t just fill prescriptions-they make critical decisions every day about which medications are safe and effective for patients. And when it comes to generics, those decisions have real consequences. A wrong substitution can lead to treatment failure, adverse reactions, or even hospitalization. With over 90% of prescriptions filled with generic drugs in the U.S., staying current isn’t optional-it’s essential.
Why Generics Knowledge Is Non-Negotiable
Generics aren’t just cheaper versions of brand-name drugs. They’re legally required to match the original in strength, purity, quality, and performance. But here’s the catch: not all generics are created equal in practice. The FDA’s Orange Book lists over 1,200 therapeutic equivalence ratings, and those ratings change monthly. A pharmacist who doesn’t track these updates might think two generics are interchangeable when they’re not.
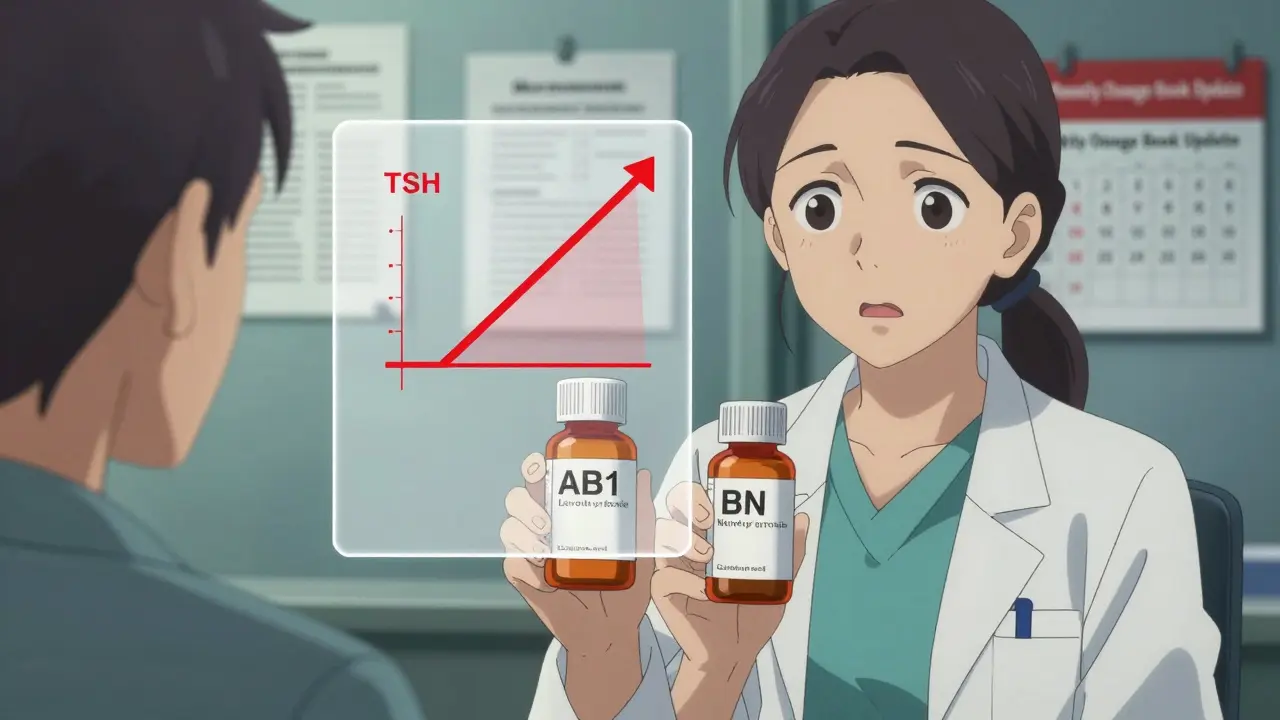
Take levothyroxine. Even tiny differences in bioavailability between brands can throw a hypothyroid patient into instability. In one case reported by a pharmacist on Reddit, switching from one generic to another without realizing they weren’t therapeutically equivalent caused a patient’s TSH levels to spike dangerously. That error was preventable-with proper education.
ACPE, the national accrediting body for pharmacy continuing education, found that 42.7% of all pharmacy malpractice claims between 2018 and 2021 involved errors related to generic substitution. That’s not a small number. It’s a systemic issue-and continuing education is the fix.
What You Need to Know About the FDA’s Rules
To approve a generic, the FDA requires manufacturers to prove bioequivalence: the generic must deliver the same amount of active ingredient into the bloodstream as the brand-name drug, within a range of 80% to 125%. That sounds precise, but it’s wide enough to matter clinically.
For drugs with a narrow therapeutic index-like warfarin, lithium, or phenytoin-that margin can be the difference between life and death. Pharmacists must know which generics are rated AB (therapeutically equivalent) and which are not. The Orange Book doesn’t just list drugs; it assigns ratings. AB1, AB2, BN, etc.-each means something different. Missing that distinction can lead to inappropriate substitutions.
And it’s not just about the FDA. The CREATES Act, passed in 2019, forces brand-name companies to provide samples to generic manufacturers so they can test for equivalence. But many generic developers still face delays or refusals. That means some generics enter the market without full testing, and pharmacists need to be aware of the risks.
State Laws Are a Maze
Every state has its own rules about when and how pharmacists can substitute generics. Texas requires pharmacists to notify prescribers before substituting narrow therapeutic index drugs. California mandates documentation of every substitution. New York requires you to submit proof of CE with your license renewal. Illinois doesn’t require submission unless audited-but you still need to keep records for five years.
And it’s getting more complicated. As of January 1, 2025, Illinois will require one hour of Cultural Competency training as part of CE. Other states now require training on opioid alternatives, biosimilars, or implicit bias. If you hold licenses in multiple states, you’re juggling different rules, deadlines, and topics. One CE course won’t cover it all.
Types of CE That Actually Work
Not all continuing education is created equal. Knowledge-based courses-where you watch a video and take a quiz-don’t stick. Pharmacists who take application-based courses, especially those using real case studies, report far fewer errors.
For example, a Pharmacist’s Letter module walked users through a case where a patient on generic levothyroxine had unexplained fatigue. The CE didn’t just explain bioequivalence-it asked: What’s your next step? Which lab values matter? How do you document your decision? That’s the kind of learning that changes behavior.
According to CE21 user reviews, application-based courses on generics score 4.7 out of 5. Knowledge-based ones? Only 3.2. The American Pharmacists Association found pharmacists who completed five or more hours of generics-specific CE annually made 37% fewer substitution errors. That’s not a coincidence-it’s proof that the right training works.
What’s Changing in 2025
ACPE announced new standards effective January 1, 2025: all generics-focused CE must now include content on biosimilars and FDA’s Risk Evaluation and Mitigation Strategies (REMS). Biosimilars aren’t traditional generics-they’re complex biologic drugs with no exact copy. Interchangeability isn’t automatic. Pharmacists need to understand the difference between biosimilar and interchangeable, and how to counsel patients.
Also, the FDA approved 983 new generic applications between January 2022 and June 2023-a 17% jump from the year before. That means new drugs, new generics, new substitution rules, and new risks. The pace of change is faster than most CE programs can keep up with.
Some providers are adapting. PocketPrep, a mobile CE platform, saw 45,000 pharmacist users in 2023, with 32% growth in generics content. CVS Health piloted just-in-time learning tools in their pharmacies-when a pharmacist selected a generic, a pop-up appeared with the latest Orange Book rating and substitution guidelines. Result? A 28% drop in errors.
How to Build Your Generics Learning Plan
You don’t have to wait for your state to mandate it. Here’s how to stay ahead:
- Track your state’s CE requirements. Know the hours, the mandatory topics, and the deadlines. Use your state board’s website-don’t rely on third-party summaries.
- Focus on application, not just facts. Choose courses with case studies. Look for ones that ask you to make decisions, not just recall numbers.
- Use the FDA Orange Book monthly. Bookmark it. Check for updates on drugs you commonly dispense. Set a calendar reminder to review it every first of the month.
- Join a peer group. Many hospital pharmacies now have monthly case review sessions focused on generics. If yours doesn’t, start one.
- Track your own errors. If you’ve ever second-guessed a substitution, write it down. Turn it into a learning moment.
On average, pharmacists spend 27.5 hours a year on CE. Only 5.2 of those are spent on generics and therapeutics. That’s not enough. If you’ve been in practice over 10 years, you need 8-10 hours of targeted generics education annually. Newer pharmacists? At least 4-6. Don’t treat CE as a checkbox. Treat it as your safety net.
Tools and Resources That Actually Help
Here are a few trusted sources that deliver real value:
- Pharmacist’s Letter - Offers free, ACPE-accredited modules on therapeutic equivalence, legal issues, and substitution ethics.
- Wolters Kluwer - Covers USP Chapters 795, 797, and 800, critical for pharmacists handling compounded generics.
- ASHP’s Online Learning Center - Has modules on biosimilars and complex generics, updated quarterly.
- FDA Orange Book - The single most important resource. Download the mobile app.
- PocketPrep - Mobile-friendly quizzes and flashcards built for busy pharmacists.
Don’t waste time on generic CE that feels like a chore. Pick the ones that make you better at your job. The goal isn’t to get a certificate-it’s to protect your patients.
Do all states require the same continuing education for generics?
No. While all 50 states require continuing education for license renewal, the topics, hours, and specific requirements vary. Some states mandate training on biosimilars, narrow therapeutic index drugs, or opioid alternatives. Illinois requires Sexual Harassment Prevention and Implicit Bias training. New York requires submission of CE certificates with renewal. Always check your state board’s official website for the most current rules.
What is the FDA Orange Book and why does it matter?
The FDA Orange Book is the official publication listing approved drug products with therapeutic equivalence evaluations. It tells pharmacists which generics are rated as AB (therapeutically equivalent) to brand-name drugs and which are not. Substituting a non-equivalent generic can lead to treatment failure or adverse effects, especially with drugs like levothyroxine, warfarin, or phenytoin. Pharmacists must check the Orange Book regularly-updates happen monthly.
Are biosimilars the same as generics?
No. Biosimilars are complex biologic drugs that are highly similar to an approved biologic (like Humira or Enbrel), but not identical. Unlike traditional generics, which are chemically identical to their brand counterparts, biosimilars are made from living cells and have slight structural differences. Interchangeability must be specifically approved by the FDA, and pharmacists must understand when substitution is allowed and when it requires prescriber authorization.
How many hours of CE do I need for generics?
There’s no universal number-it depends on your state. But experts recommend at least 4-6 hours per year for newer pharmacists and 8-10 hours for those with 10+ years of experience. The average pharmacist spends only 5.2 hours annually on generics-specific education, which is below what’s needed to stay current given the rapid pace of change.
Can I get free continuing education on generics?
Yes. Pharmacist’s Letter offers free, ACPE-accredited CE modules on therapeutic equivalence, legal issues, and substitution ethics. Other providers like ASHP and Wolters Kluwer also offer free or low-cost content. Always verify the course is ACPE-accredited or approved by your state board before completing it for credit.
What happens if I don’t complete my CE requirements?
Failing to complete required continuing education can result in license suspension or non-renewal. Some states allow a grace period with a late fee, but others impose stricter penalties, including mandatory remediation or re-examination. More importantly, skipping education puts patients at risk. Generics errors are preventable-and staying current is part of your professional responsibility.





Comments (10)