When a brand-name drug’s patent runs out, you might think generic versions hit store shelves right away. But that’s rarely the case. Even after the patent expires, it can take years before a generic version becomes available. Why? Because the path from patent expiration to actual market launch is filled with legal hurdles, regulatory delays, and strategic maneuvers by drug companies that slow down competition - and keep prices high.
The Legal Framework: Hatch-Waxman and the ANDA Pathway
The system that governs how generics enter the market was created by the Hatch-Waxman Act of 1984. This law was designed to strike a balance: protect innovation by giving brand-name companies time to recoup R&D costs, while also speeding up access to cheaper generics. It did this by creating the Abbreviated New Drug Application (ANDA) process.Under the ANDA, generic manufacturers don’t have to repeat expensive clinical trials. Instead, they prove their drug is bioequivalent - meaning it works the same way in the body as the brand-name version. That sounds simple, but getting approval isn’t just about submitting paperwork. The FDA must review the application, inspect manufacturing sites, and ensure quality standards are met. On average, this takes 25 months and 15 days from the time the application is filed.
But here’s the catch: even if the FDA approves the generic, the company can’t sell it yet. Patents and exclusivity periods still block them. The 20-year patent clock starts ticking from the day the drug is first filed, not when it hits the market. Since drug development takes 8-10 years just to get to FDA approval, most brand-name drugs only have 7-12 years of actual market exclusivity left after approval.
Patent Thickets and Layered Protection
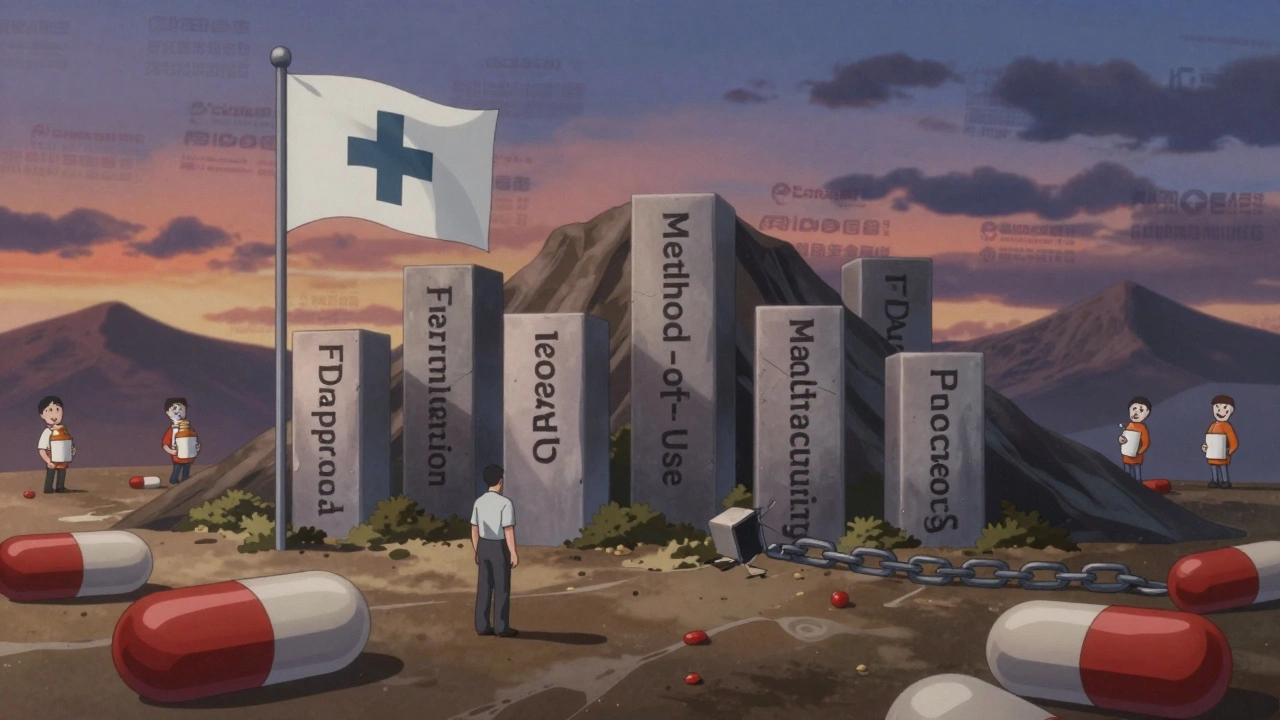
Brand-name drugmakers don’t rely on just one patent. They build what’s called a “patent thicket” - a web of overlapping protections. For a single drug, companies often list 14 or more patents in the FDA’s Orange Book. These include:- Active ingredient patents
- Formulation patents (how the drug is made - pill, capsule, extended-release)
- Manufacturing process patents
- Method-of-use patents (new conditions the drug treats)
Each of these can have its own 20-year term. Even if the main patent expires, a secondary patent on the pill coating or dosing schedule can still block generics. In 2022, the FTC found that drugs with more than 10 listed patents saw delays of 28 months longer than those with just one. Cardiovascular drugs, which often have complex formulations, averaged 3.4 years of delay after patent expiration. Dermatological products? Just 1.2 years.
These delays aren’t accidents. They’re strategic. Some companies file new patents just before the original one expires - a tactic called “patent evergreening.” A 2024 Brookings study found that 68% of top-selling drugs get at least one new patent within 18 months of the original expiring. These aren’t major innovations - often just minor changes in dosage or delivery. But they’re enough to trigger new legal battles.
The 30-Month Stay and Legal Battles
When a generic company files an ANDA, it must certify whether it believes the brand’s patents are invalid or won’t be infringed. If it says “no” - known as a Paragraph IV certification - the brand-name company has 45 days to sue. If they do, the FDA is legally required to delay approval for up to 30 months.Many assume this 30-month stay is the main reason generics are delayed. But research shows otherwise. A 2021 study in PMC found that generic drugs typically launched 3.2 years after the 30-month stay ended. That means the real bottleneck isn’t the court process - it’s the number of patents, the complexity of the drug, and the time it takes to resolve multiple lawsuits.
And here’s where things get shady. Some brand-name companies pay generic makers to delay their entry. These “reverse payment” settlements - where the brand pays the generic to stay off the market - were common for years. In 2021, the Supreme Court ruled in Amarin Pharma v. Helsinn that secret deals like these violate antitrust laws. Since then, FTC enforcement has cut these deals by nearly half. But they still happen - and they cost consumers $3.5 billion a year, according to the FTC.

The 180-Day Exclusivity Race
There’s one big incentive for generic companies to be first: 180 days of market exclusivity. The first company to successfully challenge a patent gets to be the only generic seller for six months. That’s a huge profit window.But it’s also a trap. To claim this exclusivity, the first filer must launch within 75 days of FDA approval. Many can’t - because manufacturing isn’t ready. In 2022, FDA data showed that 22% of first filers forfeited their exclusivity due to production delays. Another 10% lost it because of legal setbacks. That leaves only 68% who actually make it to market in time.
Some companies play it safe by developing multiple backup formulations. Sandoz, for example, built three to four different manufacturing processes for its generic version of Copaxone. That way, if one patent blocks one version, they can switch to another without losing time.
Why Some Generics Never Arrive - Even After Approval
Here’s the shocking part: the FDA approved 1,165 generic drugs in 2021. But only 62% reached the market within six months of approval. Why? Because approval doesn’t mean availability. Sometimes, the brand-name company controls the only supply of the reference drug needed for testing. If they refuse to sell it - a tactic called “product hopping” - the generic maker can’t even start the approval process.The CREATES Act of 2019 was passed to stop this. It forces brand-name companies to provide access to their drugs for testing. But enforcement is patchy. Many generic manufacturers still face delays just to get the sample they need to begin testing.
Complex Drugs Are the Biggest Hurdle
Small-molecule drugs - like pills for blood pressure or cholesterol - usually see generic entry within 1.5 years of patent expiration. But complex drugs? That’s a different story.Biologics - drugs made from living cells, like insulin or rheumatoid arthritis treatments - are governed by a different law: the Biologics Price Competition and Innovation Act (BPCIA). They don’t use ANDAs. Instead, they go through a slower, more complicated pathway for “biosimilars.” The average delay for biosimilars is 4.7 years after patent expiration. Even when approved, uptake is slow because doctors and patients are wary of switching.
And it’s getting harder. The FDA’s 2024 data shows that only 62% of complex generic applications met the new 24-month review target under GDUFA II. The rest are still stuck in limbo.
The Real Cost of Delay
Generics make up 92% of all prescriptions filled in the U.S. - but only 16% of total drug spending. They saved the healthcare system $373 billion in 2023 alone. But every month a generic is delayed, patients pay more.The Congressional Budget Office estimates that delaying a top-10 drug’s generic entry by one year costs Medicare $1.2 billion. For drugs that cost $500 a month, that’s tens of thousands of dollars extra per patient. And when just three companies - Teva, Viatris, and Sandoz - control 45% of the generic market, competition is already thin. Delays make it worse.
Even with AI tools being tested to speed up bioequivalence testing, and new transparency rules forcing clearer patent listings, the median time from patent expiration to generic availability is still 18 months. That’s 1.5 years of people paying brand-name prices for a drug that could be cheap.
It’s not broken. It’s rigged - by design. The system was meant to encourage innovation and competition. But today, it often protects profits over patients.
How long after patent expiration do generics usually launch?
On average, generic drugs launch about 18 months after patent expiration, but this varies widely. Simple pills may appear within a year, while complex drugs like biologics can take over four years. Delays are often caused by patent challenges, manufacturing issues, or legal settlements.
Why don’t generics launch immediately after the patent expires?
Even after a patent expires, other protections like regulatory exclusivity or additional patents can block entry. Generic manufacturers must also finish manufacturing, pass FDA inspections, and resolve any pending lawsuits. Many brand-name companies file multiple patents to delay competition, sometimes for years.
What is the Hatch-Waxman Act and how does it affect generics?
The Hatch-Waxman Act of 1984 created the ANDA process, allowing generic makers to prove bioequivalence without repeating clinical trials. It also introduced the 30-month stay and 180-day exclusivity for first filers. While it helped speed up generic access, it also gave brand-name companies tools to delay competition through patent litigation.
Can a generic drug be approved but still not available for sale?
Yes. The FDA can approve a generic drug, but if there’s an active patent, a legal injunction, or if the brand-name company refuses to provide the reference drug for testing, the generic can’t be sold. This is especially common with complex drugs where access to the original product is tightly controlled.
Do reverse payment settlements still happen?
They’re less common since the Supreme Court’s 2021 ruling, but they still occur. These deals - where a brand-name company pays a generic maker to delay launch - are now subject to antitrust scrutiny. The FTC continues to investigate and challenge these agreements, but proving them remains difficult.
How can I find out when a generic will be available for my medication?
Check the FDA’s Orange Book for patent and exclusivity information. Websites like Drugs.com or GoodRx also track generic launch dates. For complex drugs, contact your pharmacist - they often know about pending approvals or delays before the public does.




Comments (10)