When you fill a prescription, you might not think about whether the pill you’re getting is the brand name or the generic version. But behind the counter, a complex web of state rules, financial nudges, and pharmacy policies is quietly pushing doctors and pharmacists to pick the cheaper option. These are called generic prescribing incentives - and they’re one of the most effective, yet under-discussed, ways states are trying to cut drug costs without hurting patient care.
Why States Care About Generic Drugs
Generic drugs are chemically identical to brand-name drugs but cost 80% to 85% less. That’s not marketing - it’s science. The same active ingredient. Same dosage. Same safety profile. The only differences are the color, shape, and price tag. So why do people still pay more? Because brand-name companies spend millions on advertising, and patients often don’t know the difference. States stepped in because Medicaid and public health programs were bleeding money. In 2019, 46 out of 50 states had Preferred Drug Lists (PDLs) for Medicaid prescriptions. These lists say: “If you prescribe this generic instead of the brand, we’ll pay more.” If you pick the non-preferred brand? You need prior approval - or the patient pays more. It works. States that use PDLs save billions every year. But it’s not just about lists. There are other tools - and some work way better than others.The Three Big Tools States Use
There are three main ways states push for generics: copay differentials, pharmacist substitution rules, and preferred drug lists. Copay differentials are simple: charge patients less for generics. If a brand-name drug costs $30 out-of-pocket and the generic is $5, most people will choose the $5 version. In 2000, the Kaiser Family Foundation found that the gap between brand and generic copays had widened significantly - even though pharmacy profits on generics were nearly the same. That’s intentional. States figured out: if you make the patient feel the price difference, they’ll pick the generic. Pharmacist substitution laws are trickier. Some states let pharmacists swap a brand for a generic without asking the patient - that’s “presumed consent.” Others require the pharmacist to ask first - “explicit consent.” A 2018 NIH study found that presumed consent laws increased generic dispensing by 3.2 percentage points. That might not sound like much, but multiply that across millions of prescriptions, and you’re talking about $51 billion in annual savings if every state switched. Preferred Drug Lists are the most common. As of 2019, 46 states used them. But here’s the catch: they only work if the brand-name drug doesn’t offer a big enough rebate to the state. Some manufacturers pay states back huge sums to keep their drug on the list - sometimes more than the generic saves. So even if the generic is cheaper, the brand wins because the state gets paid to keep it.What Doesn’t Work (And Why)
You’d think mandatory substitution laws - forcing pharmacists to switch - would work. But they don’t. The NIH study found they had no measurable effect. Why? Because pharmacists already have a financial reason to dispense generics: they make the same profit, but the generic costs less to buy. So they’re already doing it. Adding a law doesn’t change behavior. What about paying pharmacists more to dispense generics? Also ineffective. The Department of Health and Human Services found that incentives aimed at providers - like higher dispensing fees - barely moved the needle. The real driver is the patient’s wallet. If the patient doesn’t feel the cost difference, they won’t care. And here’s another problem: some generic manufacturers are getting squeezed. The Medicaid Drug Rebate Program requires companies to pay back a portion of sales. But if the cost of ingredients goes up, or there’s a shortage, or the market gets crowded, the rebate can make the drug unprofitable. Avalere Health found five scenarios where this happens - and when it does, companies stop making the drug. Suddenly, the generic you rely on disappears. That’s not a win for cost savings. It’s a supply crisis.
How 340B and Medicare Fit In
The 340B Drug Pricing Program lets safety-net hospitals and clinics buy drugs at steep discounts - 20% to 50% off. Since 1992, it’s saved billions. But states have struggled to align their Medicaid reimbursement rules with 340B pricing. In 2016, CMS said states must reimburse pharmacies based on the actual 340B ceiling price - not some inflated number. That forced states to get more accurate with pricing. It also meant some pharmacies lost money if they didn’t adjust. Meanwhile, Medicare is watching. CMS is testing a “$2 Drug List” model for Part D - where certain low-cost generics cost $2 or less for patients. It’s voluntary, and only for Medicare, but it’s a sign of where things are headed. If it works, expect states to copy it. Why? Because it’s simple. Patients know what to expect. Pharmacies know how to process it. And it removes guesswork.Real-World Impact: What’s Changing
In the 1990s, only 33% of prescriptions were generic. By 1998, that jumped to 45%. But here’s the twist: even as more people took generics, the total spending on generics didn’t drop. Why? Because new brand-name drugs were coming out - and they were crazy expensive. So while the *share* of generics went up, the *total cost* of drugs kept rising. Today, it’s the same story. More generics are prescribed. But new specialty drugs for cancer, hepatitis, and rare diseases cost hundreds of thousands of dollars. So even with 90% of prescriptions being generics now, drug spending keeps climbing. That’s why states are doubling down on smart incentives - not just more rules. They’re learning that the best policy isn’t the one that forces change. It’s the one that makes the right choice the easiest and cheapest one.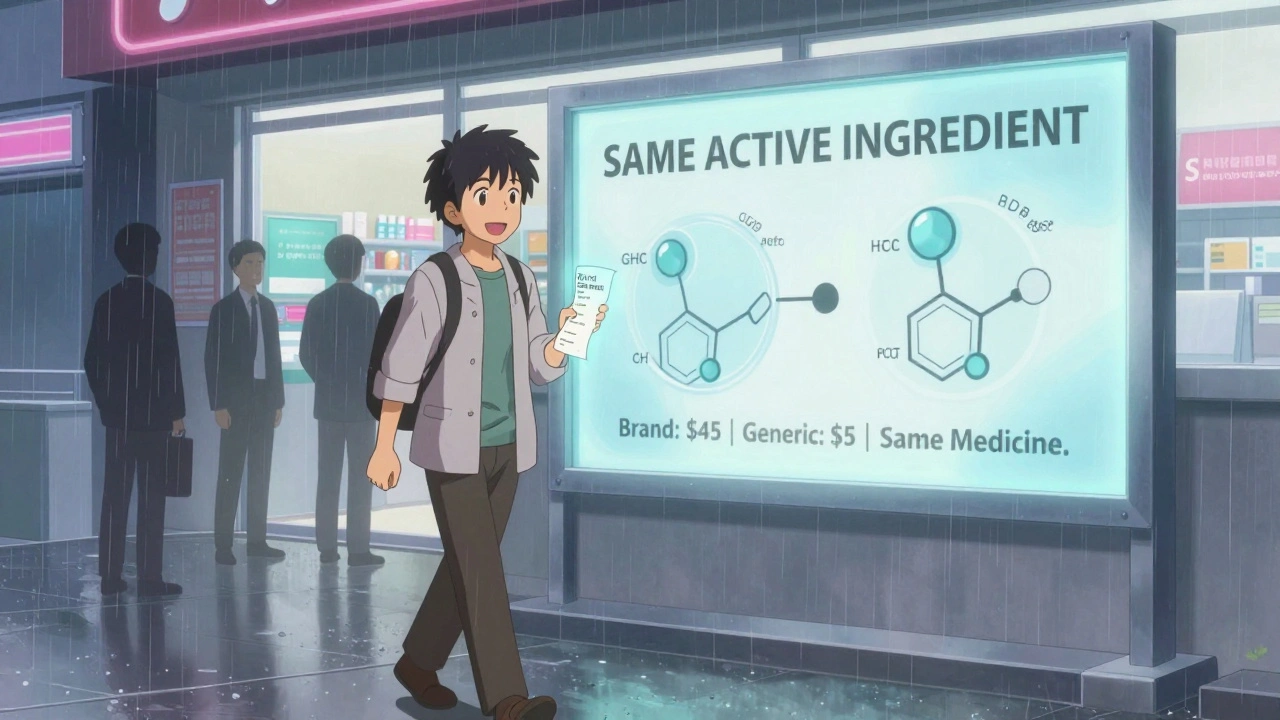
The Future: Simplicity Over Complexity
The most successful state programs aren’t the ones with the most paperwork. They’re the ones that cut through the noise. A clear copay difference. A pharmacist who can swap without asking. A list that actually saves money - not just looks good on paper. States are moving away from trying to control doctors and toward empowering patients. Because in the end, people decide what they take. If the generic is cheaper, and you know it, you’ll pick it. No law needed. The next big step? Making generic pricing as transparent as a grocery receipt. Imagine walking into a pharmacy and seeing: “Brand: $45. Generic: $5. Same medicine.” No fine print. No rebates hidden in contracts. Just the truth. That’s what the $2 Drug List is trying to build - and states are taking notes.What You Can Do
If you’re on Medicaid, Medicare, or have insurance through your employer, ask your pharmacist: “Is there a generic for this?” Don’t assume your doctor already picked it. Ask if your plan has a preferred drug list. Check your copay. If the generic is way cheaper, there’s a good chance you can switch - and save. You don’t need to be an expert. You just need to ask. Because the system is designed to make the cheap option easy - if you know where to look.Are generic drugs really the same as brand-name drugs?
Yes. By law, generic drugs must contain the same active ingredient, strength, dosage form, and route of administration as the brand-name version. They must also meet the same FDA standards for safety, effectiveness, and quality. The only differences are in inactive ingredients (like color or flavor) and price. Generics are not “weaker” or “lower quality.”
Why do some pharmacies still give me the brand-name drug even when a generic is available?
Sometimes, it’s because your doctor wrote “dispense as written” or “no substitution” on the prescription. Other times, the brand drug is on your plan’s preferred list because the manufacturer pays the insurer a big rebate. Or, the generic might be temporarily out of stock. Always ask your pharmacist - they can tell you why a substitution wasn’t made.
Do generic prescribing incentives work better for Medicaid than private insurance?
Yes, because Medicaid programs have more control over drug lists and reimbursement. States can set strict preferred drug lists, require prior authorization, and tie payments to generic use. Private insurers use similar tools, but they’re often limited by contracts with pharmacy benefit managers (PBMs) and brand-name manufacturers. Medicaid is the largest single payer in the U.S., so it has more leverage to push for savings.
What’s the difference between presumed consent and explicit consent for generic substitution?
In presumed consent states, the pharmacist can swap a brand-name drug for a generic without asking you - unless you’ve said no in advance. In explicit consent states, the pharmacist must ask you every time before switching. Studies show presumed consent increases generic use by 3.2 percentage points - a big deal when you’re talking about millions of prescriptions.
Can generic drugs disappear from the market?
Yes. Even though generics are cheaper, they can become unprofitable if the Medicaid rebate system kicks in unexpectedly - for example, if ingredient costs rise, or there’s a shortage. Manufacturers may stop making the drug because they lose money on every pill sold. That’s why some states now monitor generic supply chains closely - to avoid sudden shortages.
Is the $2 Drug List a federal program?
No, it’s a Medicare Part D model being tested by CMS. It’s voluntary and only applies to certain low-cost generic drugs for Medicare beneficiaries. But if it proves successful, states are likely to adopt similar models for Medicaid and commercial plans. The goal is to make the cheapest option simple and predictable for patients.




Comments (9)