Why Drug Companies Keep Patents Alive Long After the Original Expires
Imagine a life-saving drug that works perfectly - but the patent protecting it runs out in 10 years. Suddenly, cheaper generics flood the market. Sales drop. Profits vanish. For big pharma, that’s a nightmare. So what do they do? They don’t just wait. They build formulation patents - new patents on how the drug is mixed, packaged, or delivered - to keep competitors out even after the original patent expires.
This isn’t science fiction. It’s standard practice. Between 2015 and 2020, the FDA found that 78% of new drug applications included at least one formulation or combination patent designed to delay generic entry. These aren’t minor tweaks. They’re legal tools engineered to stretch exclusivity by 3 to 16 years. And they’re working.
What Exactly Is a Formulation Patent on a Drug Combination?
A formulation patent doesn’t protect the drug molecules themselves. That’s the job of the original composition-of-matter patent. Instead, it protects how those molecules are put together.
Think of it like baking a cake. The original patent owns the recipe for flour, sugar, and eggs. A formulation patent says: “Here’s the exact ratio - 2.3 cups flour, 1.1 cups sugar, baked at 347°F for 42 minutes - and here’s the special pan we used.” Even if anyone else uses the same ingredients, they can’t copy your exact method without breaking the law.
These patents cover:
- Specific ratios of active ingredients (e.g., 10mg of Drug A + 50mg of Drug B)
- Novel delivery systems (subcutaneous injection instead of IV)
- Modified release profiles (slow-release tablets that last 12 hours instead of 6)
- Fixed-dose combinations (two drugs in one pill)
- Unique excipients or stabilizers that improve shelf life or reduce side effects
The FDA tracks these in the Orange Book, listing them as “combination patents” or “formulation patents.” Between 2018 and 2022, these types made up 63% of all secondary patents filed. They’re not rare. They’re the backbone of how big pharma keeps revenue flowing.
How Do These Patents Survive Legal Scrutiny?
Here’s the catch: combining two known drugs isn’t automatically patentable. Under U.S. law (35 U.S.C. § 103), it’s considered “obvious” unless you can prove something unexpected happened.
The 2007 Supreme Court case KSR v. Teleflex made this harder. Courts now ask: “Would a normal scientist have thought to do this?” If the answer is yes, the patent gets rejected.
So how do companies win? They need data - hard, statistical proof.
- **Unexpected efficacy**: The combo works better than either drug alone - say, 40% more tumor shrinkage.
- **Reduced side effects**: The combination cuts nausea by 60% compared to taking the drugs separately.
- **Improved stability**: The pill lasts 3 years instead of 18 months without refrigeration.
- **Convenience**: One pill instead of three, leading to better patient adherence.
Merck, for example, spent $28-42 million extra on R&D to prove their combo drug had statistically significant benefits - with p-values under 0.01. That’s not cheap. But it’s cheaper than losing $2 billion in annual sales to generics.
And precision matters. One patent attorney on Reddit noted: “I’ve seen 10mg/50mg rejected, but 9.8mg/51.2mg approved.” Tiny changes can make the difference between a granted patent and a rejection.
Real-World Examples: The Winners and the Losers
Not every combo patent sticks. Some work brilliantly. Others collapse under legal pressure.
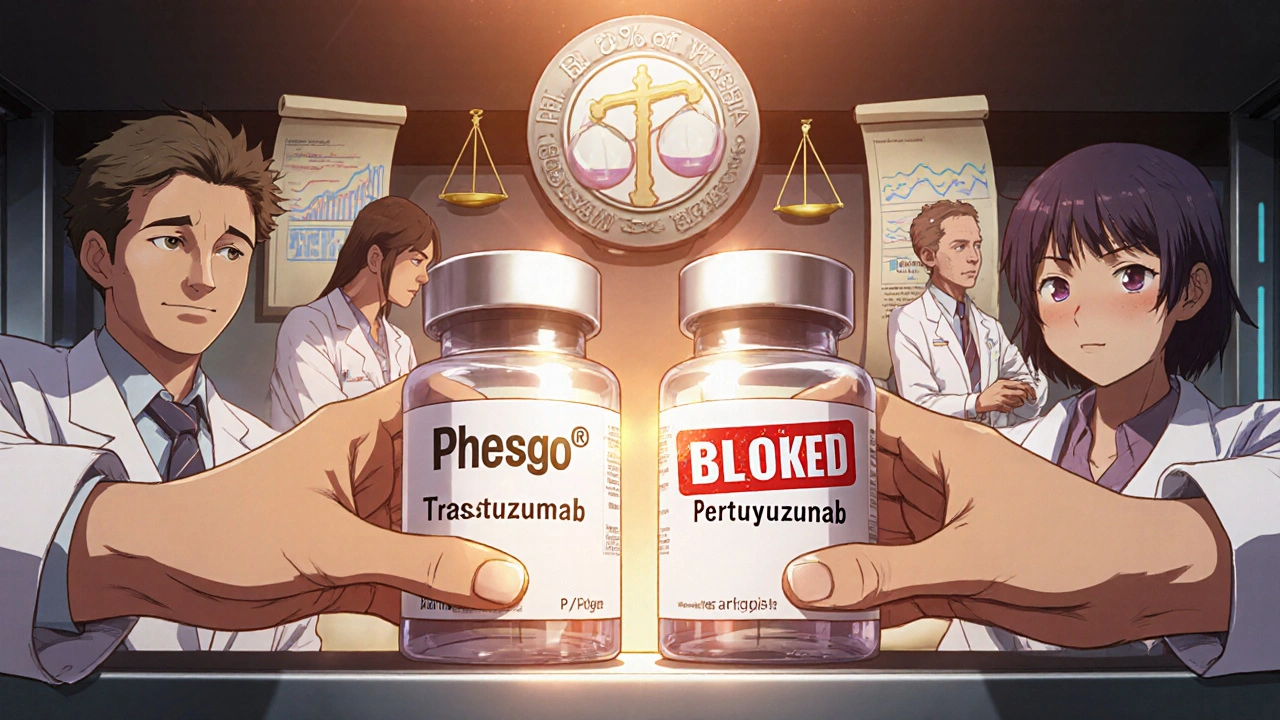
Roche’s Phesgo® is a textbook success. It combined trastuzumab and pertuzumab - two breast cancer drugs - into a single subcutaneous injection. Before Phesgo, patients needed hours-long IV infusions. Now, it’s a 5-minute shot. The FDA approved it in 2020. Even though the original patents expired, generics can’t copy Phesgo’s exact delivery system. Roche extended exclusivity by over 8 years.
AstraZeneca’s Nexium® did the same thing with esomeprazole - a purified version of omeprazole. By tweaking the molecule slightly and patenting the new formulation, they turned a $1.6 billion drug into a $189 billion revenue machine over 7 years.
But not all attempts work.
Amgen tried to patent a new auto-injector for Enbrel® - a device that simply automated the manual injection process. The court called it “obvious automation” and invalidated the patent. Amgen lost $147 million in legal fees.
And in 2021, Mylan won a case against Celgene over Revlimid®. The patent covered a specific dosage for multiple myeloma. Mylan got approval to sell a generic version for a different, non-patented use. The patent didn’t cover all uses - only one. That loophole let generics in.
The Cost: Higher Prices and Regulatory Backlash
This system isn’t free. It’s paid for by patients, insurers, and taxpayers.
According to the Congressional Research Service, secondary patents like these raise U.S. drug prices by 17-23% beyond what innovation justifies. The FTC calls it “evergreening” - a tactic to artificially extend monopolies.
Worse, some patents cover changes with no clinical benefit. The FDA found that 31% of formulation patents between 2015 and 2022 were for things like salt forms or minor excipient changes - no better efficacy, no fewer side effects. Harvard’s Dr. Aaron Kesselheim called this “patent privateering” - exploiting the system without helping patients.
And then there’s “product hopping.” That’s when a company discontinues the original drug and pushes patients onto the new patented version. In 2019, the FTC investigated oxaliplatin - a cancer drug - where the original was pulled off shelves just as generics were about to launch. Patients had no choice but to switch to the more expensive branded version.
Now, Congress is considering the Preserve Access to Affordable Generics Act, which would require companies to prove “meaningful clinical benefit” to get a new patent. If passed, it could invalidate 28% of current formulation patents.
How Generic Manufacturers Fight Back
Generics aren’t sitting still. They’re launching Paragraph IV challenges - legal notices that say, “Your patent is invalid, and we’re going to sell our version.”
In 2023, 842 such challenges were filed against formulation patents - up from 517 in 2020. Success rates? Now at 45%. Courts are getting stricter. The KSR decision gave them the tools to knock out weak patents.
And they’re getting smarter. Instead of copying the exact combo, generics design around it. They use slightly different ratios. They change the release mechanism. They target unpatented indications. One generic maker told me: “We don’t need to beat their patent. We just need to find the gap.”
Companies like Mylan, Teva, and Sandoz now have entire legal teams dedicated to finding these gaps. Their success is forcing big pharma to raise the bar - spending more on R&D, collecting better data, and filing more patents just to stay ahead.
The Future: Tighter Rules, Fewer Extensions
The era of easy evergreening is ending.
The FDA proposed new rules in May 2024 requiring “clinical superiority” for any new formulation to qualify for 3-year exclusivity. The USPTO is tightening obviousness standards. The FTC has 17 active investigations into product hopping.
As a result, the average exclusivity extension from formulation patents is dropping. From 5.3 years (2020-2023), it’s projected to fall to 3.8 years by 2025-2030.
But don’t expect this to stop. The financial stakes are too high. The global pharmaceutical market is worth $1.43 trillion. Formulation patents protect $312 billion of it - 22%. Oncology, immunology, and rare disease drugs are the biggest battlegrounds. In oncology alone, 78% of formulation patents get approved - because the clinical benefits are clearer, and patients will pay more.
Companies are adapting. Roche’s 2023 patent for a trastuzumab-deruxtecan combo with pH-sensitive release? That’s the new gold standard. It took 2.3 extra years to develop. But it’ll protect revenue for 8.5 years. That’s still worth it.
So while the tide is turning, the game isn’t over. It’s just getting harder - and more expensive - to win.
What This Means for Patients and Providers
For doctors and patients, this means confusion. Two drugs might work the same, but one is branded and costs $10,000 a month. The other is generic, but only approved for a different use. Insurance won’t cover the off-label use. So you’re stuck paying for the patent-protected version.
It also means delays. A patient might need a combo drug that’s been available overseas for years - but it’s still blocked in the U.S. because of a patent on the delivery method.
On the flip side, some of these patents have led to real improvements: fewer injections, less nausea, better adherence. Phesgo didn’t just extend exclusivity - it made treatment less traumatic for breast cancer patients.
The real question isn’t whether formulation patents are legal. They are. The question is: When does a patent stop being innovation and start being obstruction?
There’s no easy answer. But the system is changing. And patients, regulators, and generic makers are finally pushing back.
What’s the difference between a composition patent and a formulation patent?
A composition-of-matter patent protects the actual chemical structure of a drug - the molecule itself. It’s the strongest type of patent, usually lasting 20 years from filing. A formulation patent protects how that drug is delivered - the mix of ingredients, dosage form, release mechanism, or delivery device. It doesn’t protect the drug, just the way it’s packaged or administered. Formulation patents are easier to get but easier to challenge.
Can generics still enter the market if a formulation patent exists?
Yes - but not with the exact same combo or delivery method. Generics can file for approval to sell a version with different ratios, a different release profile, or for a different medical use. If they can prove their version isn’t covered by the patent, they can enter the market. Many generic companies now hire patent lawyers to find these loopholes before launching.
Why do some formulation patents get rejected?
Most rejections happen because the patent examiner decides the combination is “obvious.” If two drugs are already used together, and the new version just changes the pill shape or adds a sugar coating with no proven benefit, it won’t pass. The USPTO requires clear evidence of unexpected results - like significantly better efficacy, fewer side effects, or a major improvement in patient compliance.
How long does a formulation patent last?
Formulation patents last 20 years from the filing date, just like any other patent. But because they’re usually filed after the original patent, they often expire later. The real value comes from how long they extend market exclusivity after the original patent expires. On average, they add 3-8 years of protection, sometimes up to 16 years for drugs with layered patents.
Are formulation patents ethical?
It depends. If a formulation improves patient outcomes - like reducing side effects or making dosing easier - then yes, it’s ethical innovation. But if it’s just a minor change with no clinical benefit, designed only to block generics and keep prices high, then it’s widely seen as unethical. Regulators and courts are starting to draw that line, and companies that cross it are facing lawsuits and public backlash.




Comments (8)