When a hospital loses even one vial of oxycodone or fentanyl, the consequences aren’t just financial-they’re human. A patient might go without pain relief. Another might get a contaminated needle. And someone, somewhere, could end up addicted because a drug meant for healing ended up on the street. This isn’t a hypothetical. In U.S. healthcare settings alone, an estimated 37,000 diversion incidents happen every year. Most of them? They start with poor storage.
Storing controlled substances isn’t about locking a cabinet and calling it done. It’s about building a system where every step-from delivery to administration-is tracked, limited, and monitored. The goal? Make it nearly impossible for someone to steal, swap, or sneak out medication without getting caught. And it’s not just about following rules. It’s about protecting patients, staff, and the integrity of care itself.
What the Law Actually Requires
The Controlled Substances Act (CSA) of 1970 created a "closed system" for handling drugs like opioids, sedatives, and stimulants. That means every pharmacy, hospital, and clinic handling these drugs must be registered with the DEA and prove they have "effective controls and procedures to guard against theft and diversion." It’s not optional. And since January 1, 2025, if your facility handles more than 10kg of Schedule II substances annually, you’re legally required to have real-time inventory tracking.
The rules are clear: controlled substances must be stored in a secure location, with access limited to only those who need it. The DEA doesn’t just show up for inspections-they show up in 98% of all audits. And if they find a weak lock, an unlogged drawer, or a missing count? You could face civil penalties averaging $187,500. That’s not a fine. That’s a budget killer.
Physical Storage: Locks Aren’t Enough
Think a locked cabinet is enough? Think again. A 2022 DEA audit found that 87% of diversion risk points came from traditional locked cabinets without electronic logs. Why? Because anyone with a key can open it. No one knows who took what. No record of when. No trail.
Modern standards demand more:
- Double-locked storage: Two separate locks-one mechanical, one electronic-must be used. One person can’t open it alone.
- Access logs: Every time the vault opens, who opened it, when, and for how long must be recorded. No exceptions.
- Location matters: Storage areas must be in a secure, enclosed room-not just a closet in the pharmacy. Doors should be locked when unattended.
- No personal items: Bags, purses, coats, or backpacks are banned from medication areas. In 31% of diversion cases, stolen drugs were hidden in personal belongings.
Even small clinics should follow this. The NIH recommends bringing Schedule III-V drugs into locked storage anyway-even if state law doesn’t require it. Why? Because the risk isn’t in the schedule. It’s in the opportunity.
Automated Dispensing Cabinets (ADCs): The Gold Standard
If your facility has more than 100 beds, an automated dispensing cabinet (ADC) isn’t just smart-it’s essential. These aren’t fancy vending machines. They’re secure, computer-controlled systems that require dual authentication: a username/password + a fingerprint or badge scan.
Here’s what they do better than manual systems:
- Record every single transaction-down to the second.
- Alert pharmacists when someone takes more than usual.
- Prevent access outside shift hours.
- Reduce diversion risk by 73% when properly configured.
But here’s the catch: ADCs cost between $45,000 and $75,000 per unit. Annual maintenance? About 15% of the purchase price. That’s why smaller clinics still use manual systems. But if you go that route, you need even stricter controls.
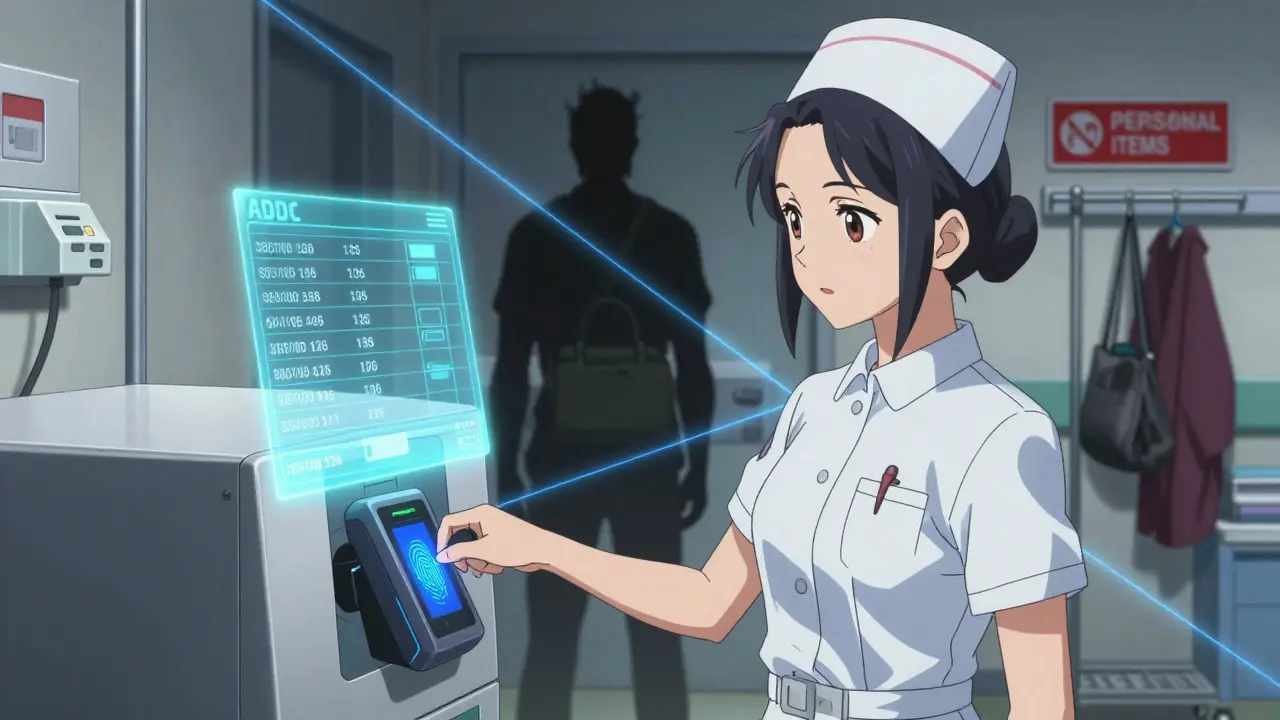
Manual Systems: How to Make Them Work
If you can’t afford ADCs, you can still prevent diversion-but you’ll need to work harder.
- Dual control: Two authorized staff members must be present for every access, refill, or count. One opens. One watches. Both sign.
- Daily audits: A pharmacist must review every controlled substance transaction. Look for patterns: Is someone always taking the last vial? Are refills happening at odd hours?
- Limit who can order: Only 2-3 people should have the authority to order bulk stock. More people = more risk.
- Train relentlessly: Staff need to know why this matters. A 2022 Mayo Clinic study found that facilities with mandatory quarterly training saw an 89% drop in diversion attempts. People don’t steal when they feel watched-and when they understand the harm.
One hospital in Texas cut diversion incidents by 74% after banning personal bags and adding dual authentication. But it took three mandatory training sessions to get staff on board. Resistance is normal. But so is change.
The Hidden Risk: Manual Transfers and Compounding
Here’s where most large-scale diversions happen: when drugs move between systems.
Think about it:
- A nurse takes a vial from the pharmacy vault to the floor.
- They mix it in a syringe.
- They document it… manually.
That’s a gap. No electronic record. No audit trail. And in 68% of major diversion cases between 2019 and 2022, that’s exactly how it happened.
Fix it:
- Use ADCs for floor stock whenever possible.
- If you must transfer manually, require two signatures and immediate documentation.
- Never let someone carry a vial out of the pharmacy without a signed requisition.
- Watch for saline flushes. In 2023, ASHP found that diverted drugs were often replaced with saline vials-making the theft invisible unless you check the weight or volume.

Monitoring and Auditing: Don’t Wait for a Theft
Good storage isn’t just about locks. It’s about watching for behavior.
- Who always works late? Who refuses to let others observe their counts?
- Is someone taking more fentanyl than the average nurse? Is a technician always "forgetting" to log a return?
Pharmacists should review logs daily. Look for outliers. A nurse who takes 10 vials in one shift when the average is 2? That’s not a mistake. That’s a red flag.
And don’t forget the human side. The ASHP guidelines say diversion prevention must include "collaborative approach, surveillance, and auditing." That means talking to staff. Building trust. Letting people report concerns without fear.
One pharmacy tech on Reddit said her facility started a "no-blame reporting" system. Within three months, three potential thefts were reported anonymously. One led to a dismissal. Two led to counseling. All three were caught before anyone got hurt.
What Happens If You Don’t Get It Right?
The cost isn’t just money.
- Legal: DEA fines, loss of license, criminal charges.
- Financial: Average $187,500 penalty per violation. Add in lawsuits if a patient is harmed-costs can hit $287,000 per incident.
- Reputational: Patients stop trusting your facility. Staff quit.
- Human: Someone you care for doesn’t get the pain relief they need. Or worse-they get infected from a reused needle.
And the pressure is rising. DEA inspections are up 37% since 2019. New rules are coming. The market for diversion prevention tech is growing at 9.3% a year. If you’re not upgrading, you’re falling behind.
Where to Start
You don’t need to fix everything tomorrow. But you need a plan.
- Map every handoff: From delivery to patient. Where are the gaps?
- Count what you have: Do you have 100 vials or 97? If you’re off, you have a problem.
- Limit access: Who really needs to open the vault? Cut it to 2-3 people.
- Install logs: Even a simple electronic keycard system is better than nothing.
- Train and talk: Explain why this matters. Not just "the rules," but "this protects our patients."
- Review weekly: Look at your logs. Ask questions. Don’t wait for the DEA to show up.
There’s no magic bullet. But there is a clear path: reduce access, increase accountability, and never stop watching.




Comments (14)